
Impact of Sterilization Conditions on the Mechanical Properties of 3D-Printed Custom Resin Solutions Custom Guide Resin
The use of implant-supported restorations has become increasingly prevalent in modern dentistry due to their superior accuracy and streamlined workflow compared to conventional restorations as evidenced, numerous studies have documented the high clinical success and long-term survival rates of implant-supported restorations [1-2]. Implant surgery is an invasive procedure during which the surgical guide comes into contact with human tissues, blood, and mucous membranes. Therefore, if the surgical guides are not appropriately sterilized, microorganisms can easily enter the surgical wound and negatively affect the surgery's success and the implant's lifespan [3]. Moreover, surgical guides should be sterilized or disinfected to avoid contamination or infection risk.
Sterilization refers to the complete destruction of all microbial life by physical or chemical processes, while disinfection eliminates microorganisms that form bacterial spores [4]. According to the CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, medical devices that come in contact with human tissue and blood are high-risk level equipment that require high sterilization methods [5]. While various sterilization methods have been used, steam autoclave sterilization is still the most commonly used sterilization method in dental practice due to its low cost, convenience, and proven sterilization effect [6-7].
This study examines the influence of steam autoclave sterilization at different temperatures on the mechanical properties (flexural modulus and flexural strength) of 3D-printed surgical guides. It further evaluates the accuracy of these guides before and after steam sterilization at 121°C (>1 bar, 20 min) and 134°C (>2 bar, 10 min). It aims to check the optimal sterilization procedure for maintaining clinical performance.
2. Materials and Methods
Eighteen standard specimens were produced using an LCD-based 3D printer (PioCreat C-01, PioCreat 3D, Shenzhen, China) with a layer thickness of 50 µm. The specimens were made with surgical guide material (Custom Guide Resin, Custom Resin Solutions, Antalya, Türkiye) to analyze the flexural properties of the materials before and after sterilization at different temperatures in a steam autoclave. After cleaning specimens with isopropyl alcohol, all printed samples were light-cured 2000 flashes (1000 flashes on the front side and 1000 flashes on the back side of the specimens) with a curing device (Otoflash G17, NK Optik, Baierbrunn, Germany) in an inert environment (with nitrogen gas). The samples were categorized into three groups (A, B, and C) with each group containing six specimens:
The samples were evaluated using a three-point flexural test with a Zwick Z250 universal testing machine (ZwickRoell, Yokohama, Japan). The applied load speed was set at 5 mm/min, and the results represent the mean values of six specimens per group.
3. Results
To better visualize the impact of sterilization conditions on the mechanical properties of the samples, the results are presented in both tabular and graphical formats. The figures below illustrate the changes in flexural modulus and flexural strength across different sterilization conditions, allowing a comparative evaluation of the material's behavior before and after autoclave sterilization.
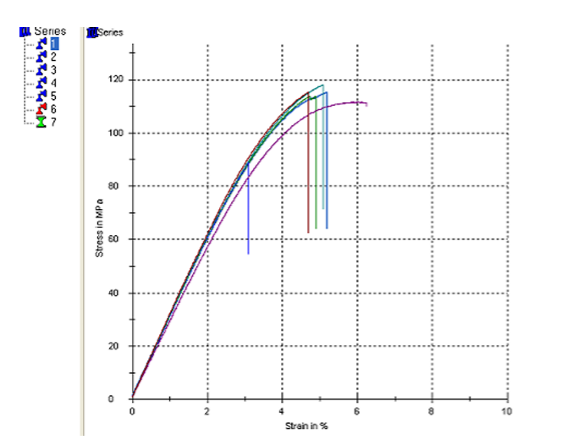
Figure 1: The graph results of the three-point bending test conducted on Group A
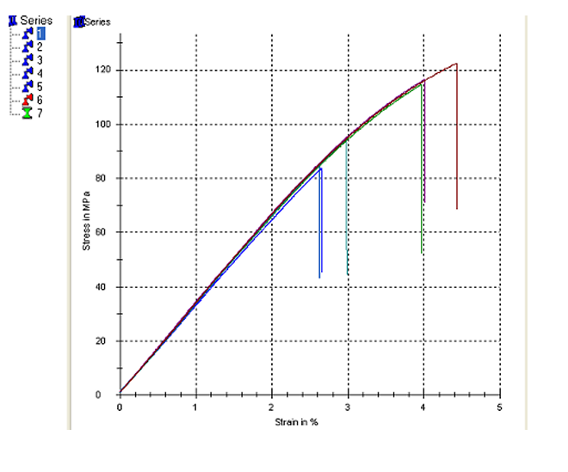
Figure 2: The results of the three-point bending test conducted on Group B
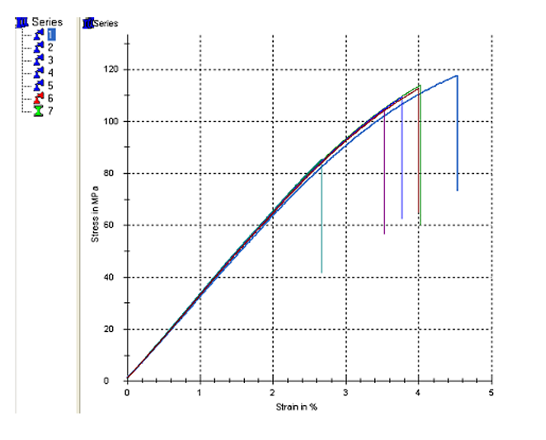
Figure 3: The results of the three-point bending test conducted on Group C
| Group | Flexural Modulus (MPa) | Flexural Strength (MPa) |
| A (Control) | 3001 | 110.4 |
| B (121°C) | 3272 | 102.7 |
| C (134°C) | 3216 | 107.2 |
Table 1: Results of all specimens
4. Conclusions
This study demonstrates the impact of steam autoclave sterilization at two different temperatures, 121°C and 134°C, on the flexural modulus and strength of Custom Resin Solutions Custom Guide Resin which used in guided implant surgery. While an increase in flexural modulus was observed, it was not statistically significant, indicating that sterilization does not adversely affect the material’s mechanical performance.
Despite a slight reduction in flexural strength, the decrease remained within clinically acceptable limits. The decrease was less pronounced at 134°C, suggesting that this higher temperature may be preferable for sterilization. However, the differences between both sterilization conditions were minor, allowing both protocols to be confidently used in clinical practice.
These findings confirm that Custom Resin Solutions Custom Guide Resin is resistant to autoclave sterilization and maintains its mechanical integrity. The material retains its hygienic and structural properties, making it a safe and durable option for guided implant surgeries.
Overall, this study provides strong scientific evidence that Custom Guide Resin can withstand sterilization without compromising its mechanical stability, reinforcing its reliability as a sterile, high-performance material in implant procedures.
References
1. Karami, D.; Alborzinia, H. R.; Amid, R.; Kadkhodazadeh, M.; Yousefi, N.; Badakhshan, S. In-Office Guided Implant Placement for Prosthetically Driven Implant Surgery. Craniomaxillofacial Trauma & Reconstruction 2017, 10 (3), 246–254. https://doi.org/10.1055/s-0036-1584891.
2. Younes, F.; Cosyn, J.; De Bruyckere, T.; Cleymaet, R.; Bouckaert, E.; Eghbali, A. A Randomized Controlled Study on the Accuracy of Free-Handed, Pilot-Drill Guided and Fully Guided Implant Surgery in Partially Edentulous Patients. Journal of Clinical Periodontology 2018, 45 (6), 721–732. https://doi.org/10.1111/jcpe.12897.
3. Sennhenn-Kirchner, S.; Weustermann, S.; Mergeryan, H.; Jacobs, H. G.; Borg- von Zepelin, M.; Kirchner, B. Preoperative Sterilization and Disinfection of Drill Guide Templates. Clinical Oral Investigations 2007, 12 (2), 179–187. https://doi.org/10.1007/s00784-007-0153-9.
4. The International Organisation for Standardization. Sterilization of Health Care Products—Vocabulary of Terms Used in Sterilization and Related Equipment and Process Standards (ISO 11139:2018). https://www.iso.org/standard/66262.html, 2024.
5. Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). https://www.cdc.gov/infection-control/hcp/disinfection-and-sterilization/index.html, 7 December 2023.
6. Han, A.; Tsoi, J. K. H.; Matinlinna, J. P.; Zhang, Y.; Chen, Z. Effects of Different Sterilization Methods on Surface Characteristics and Biofilm Formation on Zirconia in Vitro. Dental Materials 2018, 34 (2), 272–281. https://doi.org/10.1016/j.dental.2017.11.012.
7. Carvalho, F. G.; Gonçalves, L. S.; Carlo, H. L.; Soares, C. J.; Correr-Sobrinho, L.; Puppin-Rontani, R. M. Influence of Sterilization Method on the Bond Strength of Caries-Affected Dentin. Braz. Oral Res. 2009, 23 (1), 11–16. https://doi.org/10.1590/s1806-83242009000100003.